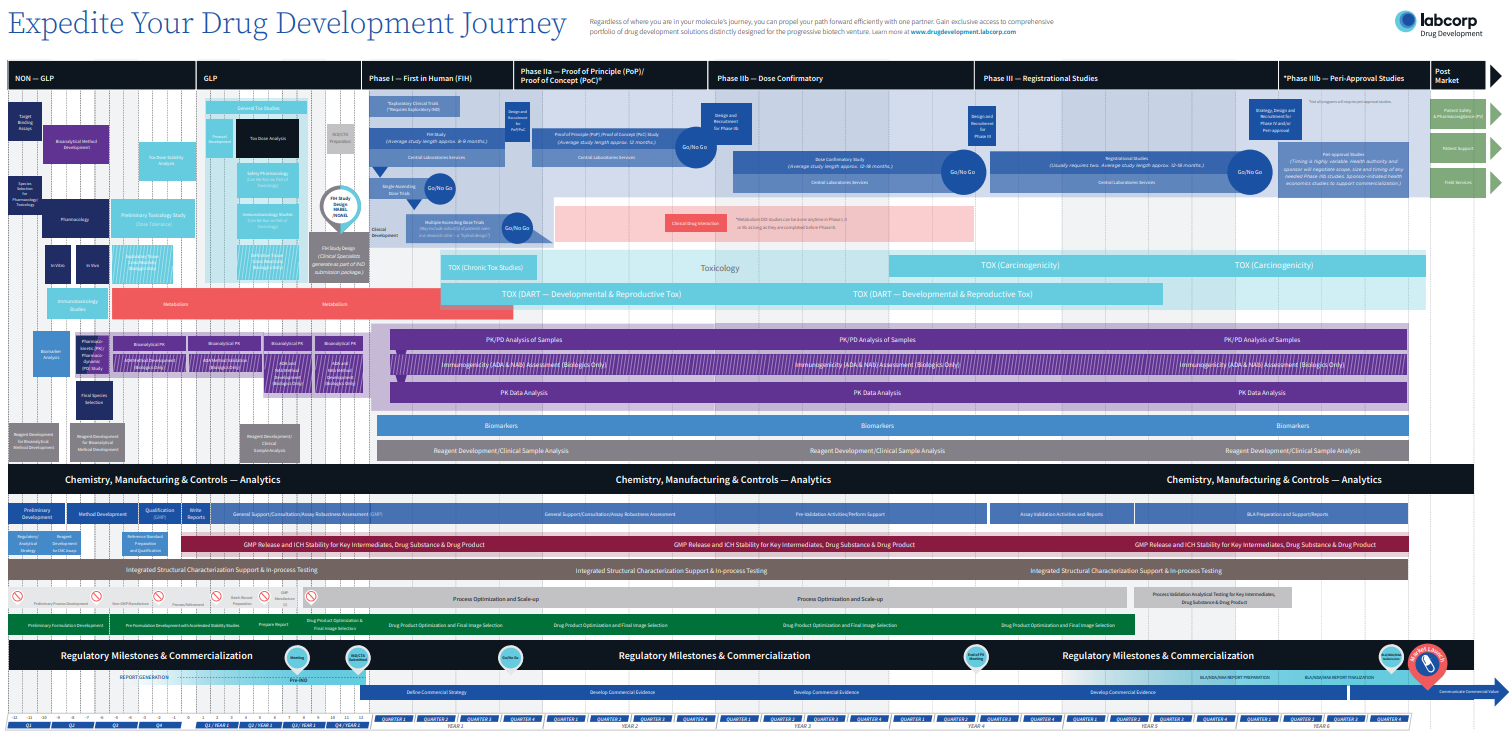
Start with the end in mind to make smarter decisions—at every stage.
With so many critical decision points in the drug development process, increased insights into early drug development efficiencies can help you uncover potential risks and opportunities, and answer the multitude of questions arising along the way:
- How can I expedite my program to respond more readily to investor and stakeholder requirements?
- How do I identify challenges early allowing me to make adjustments without losing time or money?
- How do I demonstrate that my compound will be commercially attractive to licensors or partners?
- Is this the best regulatory strategy to mitigate risk?
- How do I align my nonclinical plan with my clinical endpoints and expedite my path into first-in-human?
From the beginning, you’ll prospectively get the right strategy for your unique program. With flexible solutions and continuous support to overcome uncertainties, you’ll reach your critical decision points, faster.
Your Journey is prospectively mapped out to optimize time and maximize value:
Scientific and operational continuity — from the start.
Continuity is vital to the success of your molecule development. Early Phase Development Solutions provides you with direct access to a focused team of nonclinical and regulatory experts that will remain with you throughout your program. The result is a unified approach and consistent data package that sets you up for success.
Flexible solutions to match your unique needs.
Early Phase Development Solutions also brings a flexible, tailored approach to contracting, with options such as study-by-study or targeted milestones invoicing, all with guaranteed deliverables to meet your unique financial needs. It’s just another way you can maximize your asset’s value—and your bottom line.




